苏州微流纳米生物技术有限公司
Nanoemulsions can play an important role in many fields such as drug carriers, nanopolymerization reactors, cosmetics, and oilfield chemicals. This article will discuss the instability mechanism of nanoemulsions, the application of nanoemulsions, the influence of additives on nanoemulsions, and the permeability of nanoemulsions. A brief introduction is given in several aspects.
The unstable processes of ordinary emulsions include layering, sedimentation, flocculation, aging, coalescence and anti-equalization of the emulsion.
普通乳状液的不稳定过程包括乳状液的分层、沉降、絮凝、熟化、聚结和反相等。The above-mentioned instability processes can occur one after another or coexist at the same time. The schematic diagram of its instability mechanism is shown in the following figure
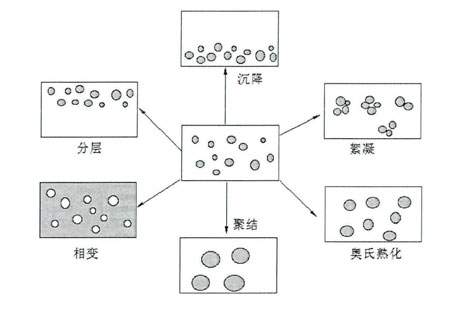
Figure Schematic diagram of the instability mechanism of
the emulsion Nanoemulsion has good kinetic stability and can maintain its appearance unchanged for a long time without obvious delamination or sedimentation. This is mainly because the droplets of nanoemulsions are very small, which can overcome some unstable factors in emulsion systems, which are mainly manifested in the following aspects:(1) The droplets of nanoemulsions are small, so the effect of gravity is small, and Brownian motion can overcome gravity, thereby preventing delamination or sedimentation;(2) Small droplets have good dispersibility, making the system not easy to flocculate;(3) Small droplets are not easy to deform and can effectively prevent fluctuations on their surfaces, which also prevents coalescence. However, in many cases, it cannot be completely prevented. However, nanoemulsions are not truly thermodynamically stable systems. Like ordinary emulsions, they have a tendency to spontaneously reduce the interface area between the dispersed phase and the dispersed medium. To sum up, the main instability mechanisms are coalescence and Aussie ripening.
(1) Liquid droplet polymerization
Liquid droplet polymerization is a phenomenon in which two droplets approach each other and collide, and the liquid film between the droplets breaks and merges into each other into large droplets. Droplet aggregation is divided into two mechanisms according to different concentrations. When the system concentration is high, droplet aggregation is dominated by rupture between liquid films; when the system concentration is low, droplet aggregation is dominated by collisions between droplets. If droplet polymerization is the main driving force for the instability of nanoemulsions, the change in droplet size follows the following equation (1-1)

Where r is the average particle diameter of the droplet at time t;ro is the initial particle diameter of the droplet; and ω is the frequency of damage to the interface film on the surface of the droplet. This formula is usually used in systems with higher concentrations. For a nanoemulsion system, if the instability mechanism is coalescence, then a straight line should be obtained corresponding to time t. Therefore, this equation can be used to determine whether the stability of the system is controlled by the phenomenon of droplet polymerization.
(2) Ostwald ripening
Ostwald phenomenon refers to the process in which small droplets gradually dissolve and deposit on large droplets over time, small droplets disappear and large droplets grow. The increase in droplet size with time can be described by the LSW(Lifshitz-Slyozov-Wagner) theory. According to the LSW theory, the Auspicious maturation rate ω of a system with solubility and droplet size r can be calculated by Equation (1-2)
![]()
Where C∞ is the solubility of an infinite droplet;y is the oil/water interfacial tension;Vm is the molar volume of the dispersed phase;D is the diffusion coefficient of the dispersed phase in the continuous phase; ρ is the phase density of the dispersed phase; R is the gas constant;T is the thermodynamic temperature. It can be seen from Equation (1-2) that for a nanoemulsion system, a straight line can be obtained by plotting the droplet radiusr3versus the time t, and the Osborne ripening rate can be calculated from the slope of the straight line.
2. The influence of additives on nanoemulsions
(1) The influence of inorganic salts
has been systematically reported. The influence of inorganic salts on various properties of non-ionic surfactants in aqueous solutions has been systematically reported. It is pointed out that the influence of inorganic salts on aqueous solutions of non-ionic surfactants mainly includes two effects: salt dissolution and salting-out, raising and lowering their cloud points respectively. Studies on the relationship between the cloud point of non-ionic surfactant systems and the phase transition temperature of emulsion systems reported that they have the same trend with temperature or added additives. Therefore, the salt that increases the cloud point of the non-ionic surfactant aqueous solution will also increase the PIT of the corresponding emulsion system, and vice versa.
(2) The influence of ionic surfactants Surfactants
play an important role in the formation of emulsions: first, they reduce the interfacial tension and promote the destruction of droplets during the emulsification process; secondly, when the interface is disturbed, they generate an interfacial tension gradient to prevent the coalescence behavior of droplets. In practical applications, a second surfactant or co-surfactant is often added to control the particle size and stability of the emulsion.
(3) Non-ionic surfactants
A small amount of anionic surfactant sodium octadecyl sulfate (SODS) and cationic surfactant sodium octadecyl bromide were added to stable oil-in-water emulsion. The results showed that both types of surfactants could greatly improve the flocculation stability of the emulsion system. Adding a small amount of cationic surfactant (CTAB) to particle-stabilized emulsions can greatly improve the stability of emulsions. Studies believe that the main role of CTAB is to:(1) promote moderate flocculation of solid particles;(2) change the wettability of particles to promote adsorption of particles on the oil-water interface; and (3) reduce interfacial tension.
3. Application of nanoemulsions
(1) Nanoemulsions as pharmaceutical delivery systems
Nanoemulsions are used as channels for drug delivery systems to pass through various systems, playing the role of active delivery and targeting. Nanoemulsions can be used for parenteral injection of some nutrients (fats, carbohydrates, vitamins, etc.), are conducive to intravenous injection, and meet the requirements for droplet sizes to be lower than lum. Nanoemulsions have the ability to control drug release and target drugs at specific locations in the body, and can be used for vaccine delivery and as gene carriers. Nanoemulsions also have obvious benefits in the application of oral drugs. The adsorption of drugs in the gastrointestinal tract is related to the size of their droplets. The smaller the droplets, the more conducive to absorption. Nanoemulsions are also used as eye delivery systems to make the drug's effects more lasting. Nano-lotion is also used in skin disinfection. Studies have shown that nano-emulsion for skin disinfection has a good killing effect on bacterial propagules and yeasts.
(2) Nanoemulsion as the reaction medium for emulsion polymerization
Using nanoemulsion as the reaction medium to synthesize nanoparticles is called nanoemulsion polymerization, also known as miniemulsion polymerization. It has been reported that nanoemulsion polymerization is compared with emulsion polymerization and microemulsion polymerization, and a mechanism for controlling the nucleation of droplets in nanoemulsion polymerization is proposed: the original size and composition of each droplet will be retained during the formation of colloidal particles, so nano-liquid droplets can be regarded as small nano-reactors.
(3) Application of nanoemulsions in cosmetics
In recent years, nanoemulsions have attracted considerable attention as a delivery system for cosmetics and personal care products. Effects such as spot fading, anti-oxidation, and acne are of positive significance for the development of curative personal care products. The researchers used palm oil nanoemulsion as the delivery system of nanocosmeceutics. The ingenious results showed that vitamin E and the surfactant Plonic F-68 (a copolymer of propylene oxide and ethylene oxide) both have an auxiliary stabilizing effect on the nanoemulsion, allowing the effective active ingredients to have better penetration on the target cells. In addition, the researchers used a high-pressure homogenization method to prepare a nano-liquid containing astaxanthin. After storing it under light and 45 ° C for one month, it was found through high performance liquid chromatography and Zeta potential analysis that the nano-emulsion has good stability for astaxanthin.
(4) Application of nanoemulsions in oil fields
Nanoemulsions in oil production, as a targeting carrier for drugs, can be used to transport scale inhibitors, water blocking agents and other treatment agents with various specific functions, which can achieve rapid and low-loss directional action; as a multifunctional treatment agent in the application of drilling fluids, it can achieve the multi-functional effect of one agent focusing on oil layer protection and combining other properties. has broad application prospects.
4. The permeable skin of nanoemulsion
consists of epidermis and dermis. The epidermis is the outer part of the skin, and the dermis is on the inner side of the epidermis. The two are tightly combined to form the prostate barrier skin between the human body and the outside world. Skin covers the outer layer of the human body and is a barrier separating the inside of the body from the external environment. It is also the outer layer of the human body that is in direct contact with the external environment. The percutaneous penetration process of cosmetics is that the functional ingredients of cosmetics are accumulated in the active skin layer after percutaneous penetration, and accumulate and act in this area, without having to penetrate through the skin and enter the systemic circulation.
In cosmetic formulations, the transdermal penetration of functional ingredients is generally promoted through two aspects: one is to promote the penetration and absorption of functional ingredients in the skin through penetration promoters such as azone; the other is to control the release of functional ingredients through excipients such as microemulsions, liposomes and nanoemulsion technologies. The application of penetration enhancers is the development of modern medical technology in cosmetics. However, potential preservatives and allergenic ingredients in cosmetic formulations can also penetrate into the skin at the same time, causing adverse reactions. At present, nanoemulsion technology has been continuously introduced into modern cosmetics as a controlled release system for functional ingredients.
more details, please contact
 | manager Wang |