苏州微流纳米生物技术有限公司
Myocardial infarction (also known as myocardial infarction, myocardial infarction) refers to myocardial necrosis caused by acute, persistent ischemia, and hypoxia (coronary artery dysfunction). The consequences of myocardial infarction are serious, and restoring myocardial blood supply in a short period of time is an effective method to rescue dying myocardium. In recent years, studies have shown that atorvastatin calcium can protect myocardial damage during ischemic injection, reduce ventricular remodeling, and improve cardiac function. Nanoliposomesare a new colloidal drug delivery system that has the advantages of increasing drug solubility, improving drug targeting, delaying drug release, and reducing drug dose toxicity. In this study, atorvastatin calcium was used as a model drug to prepare atorvastatin calcium nanoliposomes, and its protective effect on cardiac ischemic injury in rats.
This article will introduce the process flow of "hot melt emulsification-high pressure homogenization technology-liposome extrusion technology" to prepare atorvastatin calcium nanoliposomes, evaluate the particle size distribution and encapsulation efficiency of the product, optimize the formulation of atorvastatin calcium nanoliposomes through central composite design-response surface methodology, and evaluate the surface morphology, particle size distribution, Zeta potential, and in vitro drug release of nanoliposomes. To investigate the protective effect of atorvastatin calcium nanoliposomes.
Instruments and Materials
LC-10AD high performance liquid chromatograph (Shimadzu Corporation, Japan) ;
NanoGenizer 20K high-pressure homogenizer (Suzhou Microfluidic Nanobiotech Co., Ltd.);
Nicomp380 laser particle sizer (Suzhou Microfluidic Nanobiotech Co., Ltd.) ;
Genizer Extruder online liposome extruder (Suzhou Microfluidic Nanobiotech Co., Ltd.)
JEM-1200EX transmission electron microscope (Japan Electronics Company) ;
FA1004N electronic analytical balance (Henan Brother Instrument Equipment Co., Ltd.) ;
Model TGL-16G benchtop centrifuge (Shanghai Anting Scientific Instrument Factory);
atorvastatin calcium bulk drug substance; glycerol behenate; polyethylene glycol oleate; soybean lecithin.
Methods and Results
Preparation of atorvastatin calcium nanoliposomes by hot melt emulsification-high pressure homogenization technology:
① Place the prescribed amount of soybean phospholipid and poloxamer 188 in a conical flask, add 40 ml of distilled water, and place them in a constant temperature water bath at 75 ℃, and use magnetic stirring speed of 1500 r per minute to completely dissolve them to obtain the aqueous phase;
② Place the prescribed amount of glycerol monostearate, polyethylene glycol oleate and atorvastatin calcium in a conical flask, and use magnetic stirring speed of 1500 r per minute to completely dissolve them to obtain the oil phase;
③ Under stirring in a high-speed shear dispersion machine ( 10000 r/min) , slowly add the drug-containing oil phase into the water phase, and continue to shear and emulsify for 10 minutes to prepare colostrum;
④ The colostrum was homogenized 4 times under a pressure of 200 MPa through a NanoGenizer 20K high-pressure homogenizer, diluted with distilled water to constant volume, cooled to room temperature, and passed through a 100-nm membrane of a Genizer Extruder online liposome extruder to prepare atorvastatin calcium nanostructured lipid carriers.
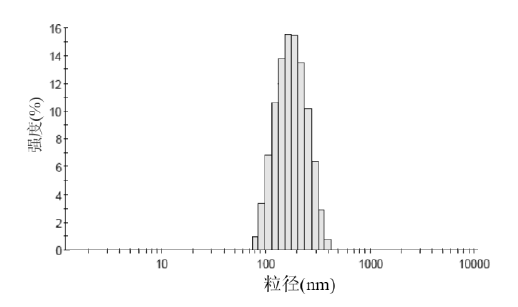
Figure 1.& nbsp; Particle size distribution of atorvastatin calcium nanostructured lipid carriers
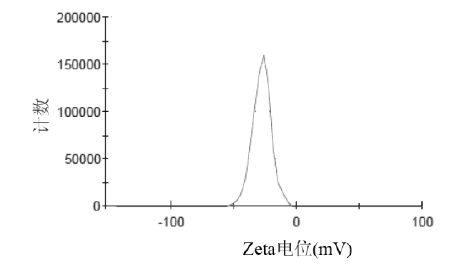
Figure 2.& nbsp; Zeta potential of atorvastatin calcium nanostructured lipid carriers
Atorvastatin calcium nanoliposomes were diluted with distilled water, and their particle size distribution and Zeta potential were measured using a nicomp380 laser particle sizer . The particle size of atorvastatin calcium nanoliposomes has a single peak distribution, with an average particle size of ( 122.6 ± 39.7) nm and a Zeta potential of (-26.4 ± 3.7) mv. The surface of nanoparticles usually carries negative charges, and the higher the absolute potential of Zeta, the more conducive the stability of the system. The atorvastatin calcium nanoliposomes prepared in this study have a high absolute Zeta potential value, which may be due to the molecular polarization and the adsorption of the emulsifier molecules on the charge in water, forming an ionic-like electric double layer structure, resulting in a higher Zeta potential value. At the same time, the existence of long polyethylene glycol chains in polyethylene glycol oleate glycerol ester added to the system will hinder the aggregation of nanoparticles, and together with electrostatic repulsion will achieve the purpose of stabilizing the system. In this study, the results of rat myocardial ischemia/reperfusion experiments proved that preparation of atorvastatin calcium into nanostructured lipid carriers can significantly improve the protective effect of atorvastatin calcium on myocardial ischemia injury in rats.