苏州微流纳米生物技术有限公司
Dexamethone (dexamethasone) is a common anti-allergic drug that is clinically suitable for the treatment of asthma, skin diseases, various malignant lymphoma and other diseases, and has significant effects on the treatment of arthritis. At present, the main clinical applications are dexamethasone palmitate fat emulsion injection and dexamethasone acetate suspension injection. Dexamethasone acetate suspension can only be injected intramuscularly and is not suitable for intravenous injection. It is used for injection into the joint cavity during the treatment of arthritis and is easily deposited on the periosteum. However, palmitate-esterified dexamethasone, dissolved in soybean oil to make fat emulsion injection, is not easy to deposit on the periosteum. Moreover, drug-loaded fat emulsion is easy to concentrate on inflammatory sites after intravenous injection, which can improve anti-inflammatory accuracy and activity and reduce adverse reactions.
Instruments and Materials
NanoDeBEE high-pressure homogenizer (Suzhou Microflow Nanobiotech Co., Ltd.)
IKAT18 ULTRA-TURRAX high-speed shear machine (IKA Group Corporation of Germany)
PF-101T heat collection constant temperature magnetic stirrer (Yingyu Yuhua Instrument Factory, Gongyi, Henan)
GL-22M high-speed refrigerated centrifuge (Nanjing Laibu Technology Industrial Co., Ltd.)
BP211D electronic balance (Germany's Sedolis Group)
PHS-3C acidity meter (Shanghai Weiye Instrument Factory)
Zeta Sizer 2000HS laser particle size analyzer (Malvern Company, UK)
Dexamethasone palmitate API, soybean oil for injection, egg yolk lecithin E80, sodium oleate, glycerol for injection
Experimental Method
Ø Drug-oil phase preparation: Add the prescribed amount of dexamethasone palmitate into soybean oil and stir to dissolve, then weigh the prescribed amount of lecithin and add it into the above oil phase, protect with nitrogen, control the water bath temperature, heat and stir to dissolve it;
Ø Preparation of aqueous phase: respectively add the prescribed amount of glycerol and sodium oleate to appropriate amount of water for injection, control the water bath temperature, heat and stir to dissolve;
Ø Preparation of drug-containing colostrum: start the high-speed shearing and dispersing machine, adjust the speed of the shearing knife to 20,000 rpm, control the water bath temperature, slowly add the drug-containing oil phase into the water phase, and continue to shear and emulsify for 10 minutes to prepare drug-containing colostrum;
Ø Preparation of dexamethasone palmitate fat emulsion: Adjust the pH of drug-containing colostrum to 7.5 - 8.5, dilute with water for injection to constant volume to the full volume, transfer to a NanoDeBEE high-pressure homogenizer for homogenization, and adjust the homogenization pressure and number of homogenization to prepare dexamethasone palmitate fat emulsion;
Ø Subpack the fat emulsion into ampoules, flush with nitrogen, seal, sterilize it under high pressure at 121 ° C for 15 minutes, and quickly cool down in a cold water bath.
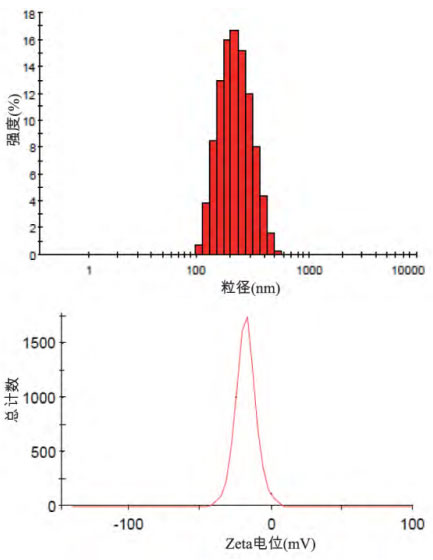
photos by Determination of particle size distribution and Zeta potential of dexamethasone palmitate fat emulsion injection
Experimental summary
After gradient transformation experiments of preparation process parameters, it was found that the key process parameters for preparing dexamethasone palmitate fat emulsion were as follows: preparing drug-containing colostrum in a water bath at 70℃, and homogenizing the drug-containing colostrum in a high-pressure homogenizer. The homogenization pressure was 1500bar, and the number of homogenizations was 4 times. Sterilize by autoclave at 121℃ for 15 minutes, and cool for later use. The particle size distribution and Zeta potential measurement of three batches of dexamethasone palmitate fat emulsion were continuously prepared according to the above key process parameters.